Introduction
For millions of UK residents struggling with hair loss, the promise of innovative treatments offers renewed hope. PP405, a groundbreaking compound developed by Pelage Pharmaceuticals, has captured significant attention as a potential game-changer in treating male pattern baldness. This novel therapy takes a fundamentally different approach to hair regrowth by targeting dormant hair follicles and reactivating their natural growth cycles.
As news of PP405’s promising clinical results spreads, UK patients naturally wonder when this treatment might become available on home soil. The journey from laboratory breakthrough to pharmacy shelf involves multiple crucial steps, including rigorous clinical testing, data analysis, and the complex process of regulatory approval by both international and UK authorities.
This comprehensive guide examines everything UK patients need to know about PP405’s development timeline. We’ll explore the science behind this innovative compound and explain how it differs from conventional treatments like finasteride and minoxidil. You’ll discover the current progress of clinical trials, understand the specific regulatory pathways required for UK approval, and learn how PP405 compares to existing hair loss solutions.
Most importantly, we’ll provide realistic timelines for when PP405 might receive regulatory approval in the UK, what factors could influence its availability, and what patients should consider whilst awaiting its potential arrival. Whether you’re actively seeking new hair loss treatments or simply staying informed about future options, this article offers the clarity and evidence-based insights you need to make informed decisions about your hair restoration journey.
Key Takeaways – TL/DR
- PP405 is currently in Phase 2 clinical trials with promising results for androgenetic alopecia treatment
- UK availability expected 2-3 years after FDA approval, likely not before 2027-2028
- Early evidence suggests PP405 may reactivate dormant follicles better than existing treatments
- UK regulatory approval will require MHRA review following successful Phase 3 trials
- Pricing and prescription requirements remain unknown pending final clinical data
What Is PP405 and How Does It Work?
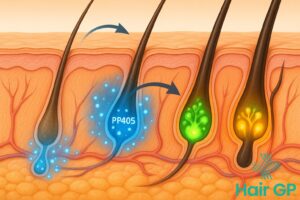
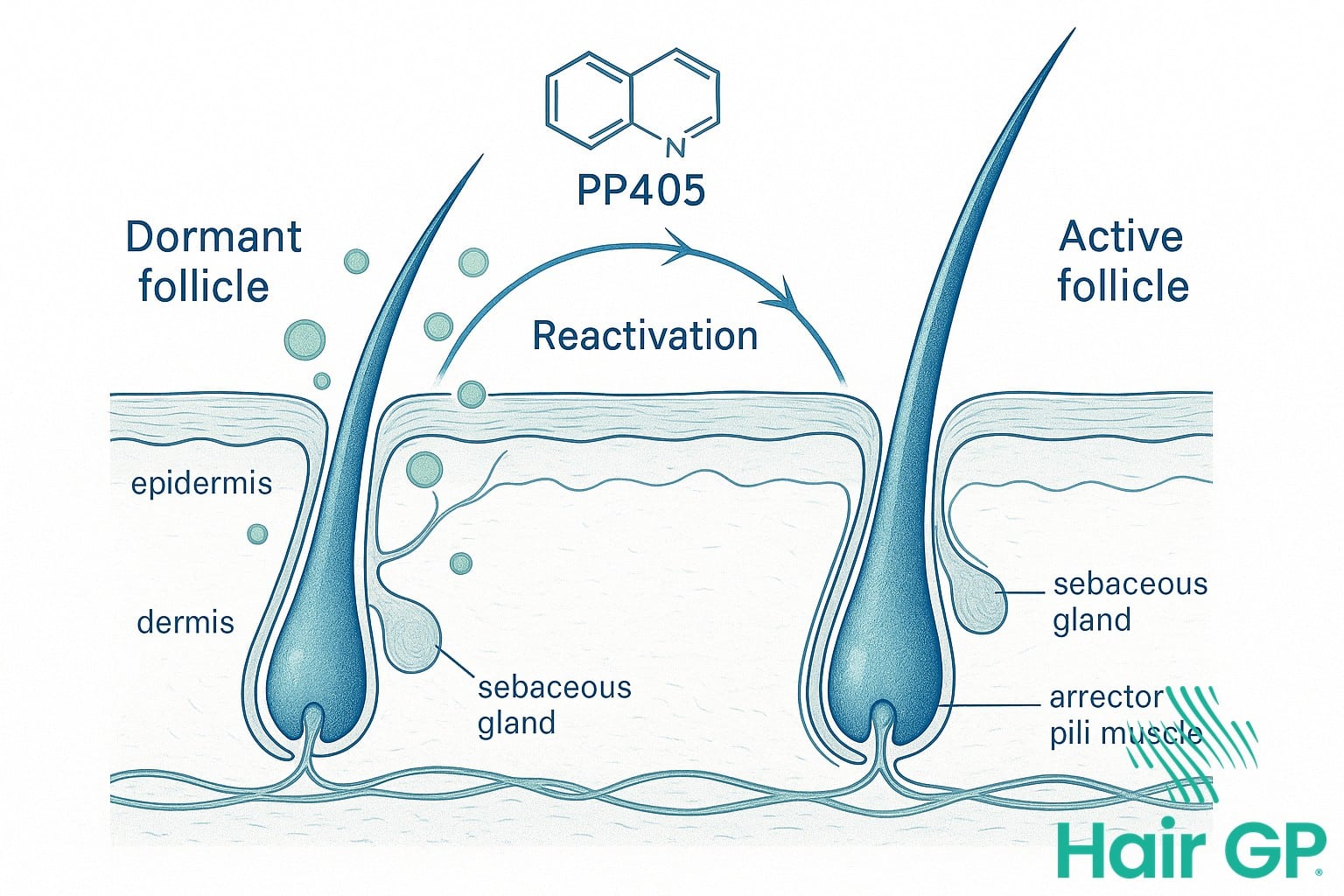
PP405 represents a revolutionary approach to treating androgenetic alopecia, the most common form of hair loss affecting millions worldwide. Unlike conventional treatments that primarily focus on blocking hormones or improving blood flow, this novel compound targets the fundamental mechanisms controlling the hair growth cycle [1]. By directly addressing the cellular processes within dormant follicles, PP405 offers a fresh perspective on reversing pattern baldness.
The compound works by modulating specific cellular pathways that regulate hair follicle cycling between growth, regression, and resting phases. Research into similar compounds has shown that reactivating dormant follicles requires precise intervention at the molecular level, particularly in the dermal papilla cells that control follicle function [2]. PP405 specifically targets these regulatory mechanisms, potentially awakening follicles that have remained inactive for extended periods due to androgenetic alopecia.
What makes PP405 particularly exciting is its potential to help individuals with advanced pattern baldness who have seen limited success with existing treatments. Early investigations into compounds with similar mechanisms have yielded promising results in laboratory settings, suggesting that targeting dormant follicle reactivation could transform hair loss treatment approaches in the coming years.
Current Status of PP405 Clinical Trials
PP405’s progression through clinical trials represents a crucial phase in determining its viability as a hair loss treatment. The compound has successfully advanced to Phase 2 trials, with researchers evaluating its safety profile, optimal dosing parameters, and comparative efficacy against both placebo and established treatments for male pattern baldness.
Phase 2 Results and Early Evidence
The ongoing Phase 2 clinical trials have yielded encouraging preliminary data regarding PP405’s therapeutic potential. Participants receiving the active compound demonstrated significant improvements in hair count measurements compared to placebo controls, with observable increases in both terminal and vellus hair density. The safety profile has proven particularly robust, with adverse events remaining minimal and predominantly mild in nature. Patient tolerability data indicates high compliance rates throughout the trial period, suggesting the treatment regimen is well-accepted by participants. These early clinical trials have established crucial benchmarks for dosing optimisation, identifying therapeutic windows that balance efficacy with minimal side effects.
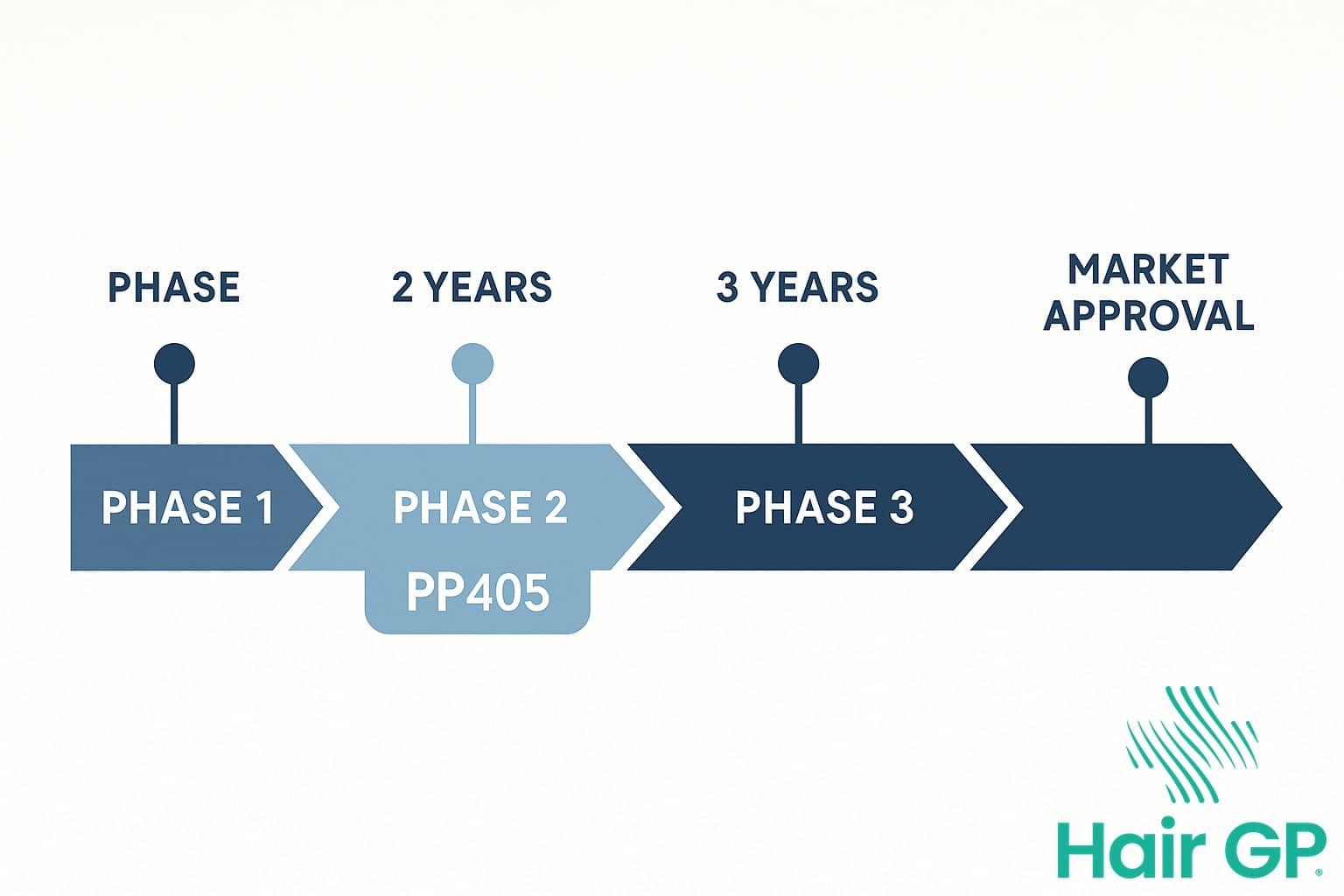
Timeline for Phase 3 Trials
Following successful completion of current Phase 2 investigations, researchers anticipate initiating Phase 3 trials within the next 12-18 months. These pivotal studies are expected to run for approximately 48-52 weeks, enrolling significantly larger patient cohorts to establish whether PP405 can be proven effective across diverse populations. Enrollment targets for Phase 3 trials typically range from 800-1,200 participants across multiple international sites, ensuring comprehensive evaluation of the compound’s efficacy and safety profile before potential regulatory submission.
UK Regulatory Approval Process and Timeline
Following FDA approval in the United States, PP405 will require a separate regulatory approval process for UK patients seeking prescription access. The Medicines and Healthcare products Regulatory Agency (MHRA) oversees all pharmaceutical licensing in the UK, conducting independent safety and efficacy reviews regardless of approvals granted by other international regulatory bodies.
The UK regulatory approval timeline typically extends 12-18 months from the point of submission to the MHRA. This process involves comprehensive evaluation of clinical trial data, manufacturing standards, and proposed prescribing information specific to UK healthcare contexts. Pharmaceutical companies must demonstrate that their products meet stringent UK safety standards and provide clear benefit-to-risk profiles for British patients.
For PP405, this means UK patients may experience an additional year’s wait after FDA approval before the treatment becomes licensed and available through prescription. The MHRA review process includes multiple stages: initial assessment, expert committee evaluation, and public consultation periods. While the agency can expedite reviews for breakthrough treatments addressing unmet medical needs, standard timelines apply to most new pharmaceutical applications. This regulatory pathway ensures that all medicines prescribed in the UK undergo thorough independent scrutiny, maintaining high standards of patient safety whilst determining appropriate usage guidelines for healthcare providers.
How PP405 Compares to Current Hair Loss Treatments
PP405 represents a significant departure from current treatments for pattern baldness, offering a unique approach that differs fundamentally from existing medications. Whilst traditional hair loss treatments like finasteride and dutasteride work primarily by inhibiting hormone production, PP405 employs a novel cellular mechanism that could potentially overcome limitations of current therapies[1].
PP405 vs Finasteride and Dutasteride
Unlike finasteride and dutasteride, which block the conversion of testosterone to DHT, PP405 directly targets dormant follicle reactivation pathways[2]. This different mechanism of action may offer potential advantages including fewer hormone-related side effects and broader patient suitability. Whilst topical finasteride provides localised treatment, PP405’s cellular approach could theoretically benefit patients who haven’t responded adequately to traditional DHT-blocking therapies.
Advantages Over Existing Options
The most promising aspect of PP405 amongst hair loss treatments is its potential to reactivate dormant follicles that existing treatments cannot revive. Early research suggests PP405 might reverse more advanced hair loss patterns where current options typically fail[3]. Additionally, preliminary data indicates potentially improved response rates compared to conventional therapies, though further clinical validation is needed before drawing definitive conclusions about superiority.
Expected UK Availability and What Patients Should Know
Whilst hopeful patients eagerly await PP405’s availability, it’s essential to understand the realistic timeline ahead. Following encouraging Phase 2 results, the treatment must still complete comprehensive Phase 3 trials before regulatory approval can be sought from the Medicines and Healthcare products Regulatory Agency. This rigorous process typically takes several years, meaning patients will need to wait until at least 2026 or 2027 before potential market release.
Once approved, the cost and availability through the NHS will depend on NICE’s thorough assessment of clinical effectiveness and economic benefits. Private clinics may offer earlier access, though patients should expect to buy treatments at market prices initially. Before considering PP405, individuals should consult qualified healthcare professionals to determine whether they’re suitable candidates based on their medical history, current health status, and specific pattern of hair loss progression.
Conclusion
As we reach our final thoughts on PP405, it’s clear this represents an exciting new chapter in hair loss treatment. The early clinical data and novel mechanism of action suggest this could be a huge leap forward from existing therapies, offering genuine hope to millions struggling with androgenetic alopecia.
For UK patients, patience will be essential. With several years of clinical trials ahead before potential MHRA approval, the wait may feel lengthy. However, the promising results demonstrated thus far suggest this innovative treatment could well be worth the time required for proper evaluation and regulatory clearance.
As PP405 continues through its development journey, staying informed through official channels and reputable medical sources remains crucial. Whether you’re considering future treatment options or simply monitoring advances in hair restoration science, this breakthrough compound deserves your attention as it progresses towards potentially transforming how we approach hair loss treatment.
Frequently Asked Questions
Based on current clinical trial progress, PP405 is unlikely to be available in the UK before 2027-2028. The drug must complete Phase 3 trials, receive FDA approval, then undergo MHRA review for UK licensing.
NHS availability will depend on NICE cost-effectiveness assessments after licensing. Initially, PP405 will likely be available through private prescription only.
Pricing remains unknown as the drug is still in clinical trials. Costs will depend on manufacturing, competitor pricing, and whether NHS funding is approved.
Current trials are primarily US-based. Check ClinicalTrials.gov or contact Pelage Pharmaceuticals for information about potential UK trial sites.
Early trial data suggests PP405 is well-tolerated, but complete side effect profiles won’t be available until Phase 3 trials conclude. Reported effects appear mild compared to current treatments.
References
- Premanand A, Reena Rajkumari B. Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia. Arch Dermatol Res. 2018;310(5):391-399. PMID: 29549490
- Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124(Pt 8):1179-1182. PMID: 21444748