Introduction
For millions of people worldwide, hair loss represents more than just a cosmetic concern—it’s a deeply personal challenge that affects confidence, self-image, and quality of life. Traditional treatments have offered limited success, often requiring continuous use with modest results. Now, a groundbreaking discovery from UCLA researchers promises to transform the landscape of hair restoration entirely.
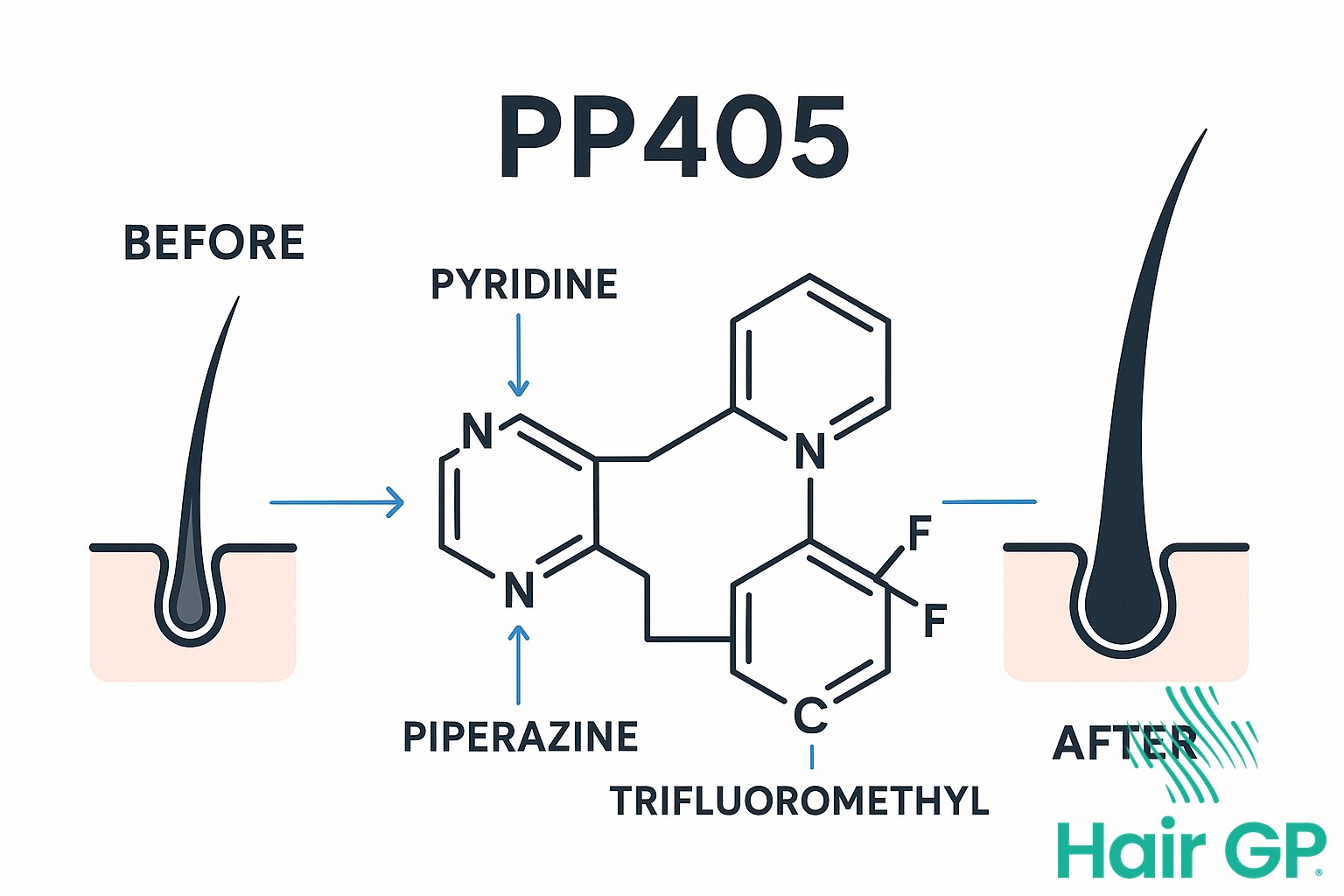
PP405, the revolutionary hair restoration treatment that could change everything, represents a fundamental shift in how we approach hair loss. This innovative new molecule, developed by Pelage Pharmaceuticals following years of meticulous research, works through mechanisms previously unexplored in hair restoration science. Unlike conventional treatments that merely slow hair loss or require surgical intervention, PP405 actively awakens dormant hair follicles, potentially reversing the balding process itself.
What makes this discovery particularly exciting is its novel approach to the biological roots of hair loss. The UCLA researchers behind PP405 have identified specific cellular pathways that, when activated, can restart the natural hair growth cycle in follicles that have ceased functioning. This protein-based treatment offers hope to those who have found limited success with existing options.
In this comprehensive article, we’ll explore the fascinating science behind PP405, examining how it differs from current treatments and what makes it so promising. We’ll delve into the clinical trial data that has generated such excitement in the medical community, and provide a detailed comparison with existing hair restoration methods. Whether you’re personally affected by hair loss or simply interested in cutting-edge medical breakthroughs, this analysis will provide valuable insights into what could be the most significant advance in hair restoration treatment in decades.
Key Takeaways – TL/DR
- PP405 is a revolutionary new molecule that activates follicle stem cells to promote natural hair regrowth
- First human trials showed statistically significant results in just one week
- Unlike current treatments, PP405 works at the molecular level to redefine how we approach baldness
- Pelage Pharmaceuticals plans further trials with potential market availability within 3-5 years
- The treatment could offer hope to millions suffering from various forms of hair loss
What Is PP405 and Why Does It Matter?
PP405 represents a revolutionary new molecule that targets the fundamental mechanisms of hair growth at the cellular level. Uncovered by researchers at UCLA, this protein-based drug activates dormant follicle stem cells through a novel molecular pathway, offering hope for millions suffering from hair loss conditions.
The Discovery at UCLA
The breakthrough emerged from UCLA’s regenerative medicine laboratory, where a multidisciplinary team of molecular biologists and dermatologists investigated cellular signalling pathways in hair follicles. Working over several years, the research team identified a previously unknown protein interaction that directly influences stem cell behaviour within hair follicles. Their initial findings revealed that PP405 could reactivate dormant follicles by targeting specific molecular receptors, essentially ‘waking up’ cells that had ceased producing hair. The discovery marked a significant departure from traditional approaches, which typically focus on extending the growth phase of existing hair rather than regenerating new follicles.
How PP405 Differs from Current Treatments
Unlike conventional therapies such as minoxidil or finasteride, which work by improving blood flow or blocking hormones, PP405 operates through direct molecular targeting of follicle stem cells. This protein-based drug binds to specific receptors on stem cells, triggering a cascade of regenerative signals that promote natural hair regrowth. The treatment’s mechanism bypasses the limitations of current options by addressing the root cause of follicle dormancy rather than merely managing symptoms. Most significantly, PP405 stimulates the body’s own regenerative processes, potentially offering more sustainable and comprehensive results than existing treatments that require continuous application to maintain their effects.
The Science Behind PP405: Activating Dormant Follicles
The science behind PP405’s remarkable hair restoration capability lies in its unique ability to reawaken dormant follicle stem cells. This breakthrough treatment works at the cellular level, targeting the biological mechanisms that control hair growth and regeneration throughout the scalp.
Understanding Follicle Stem Cell Activation
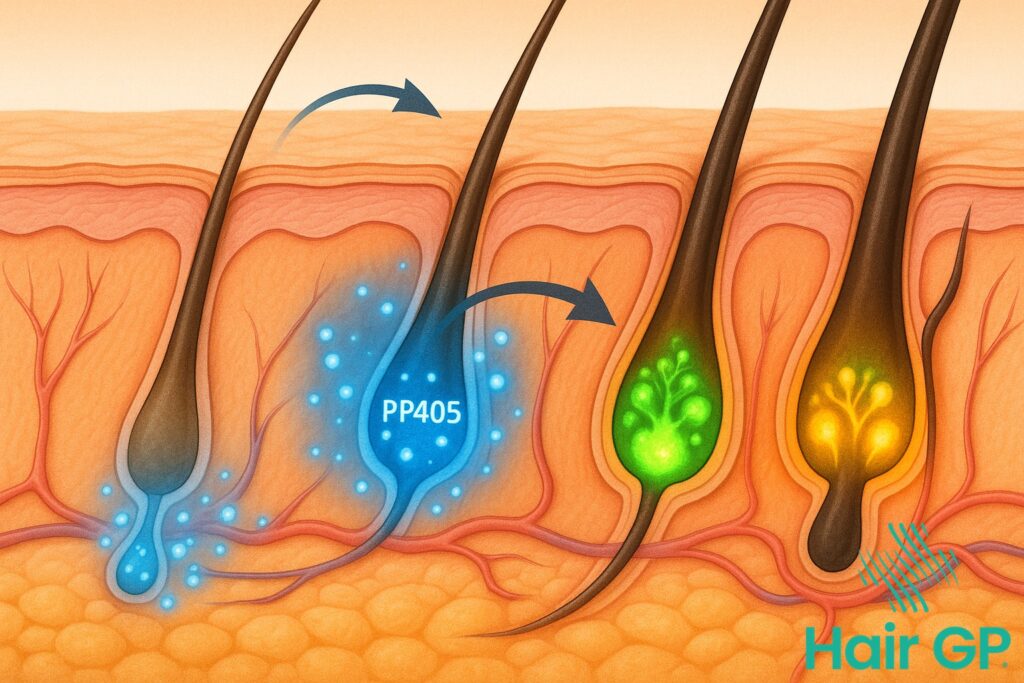
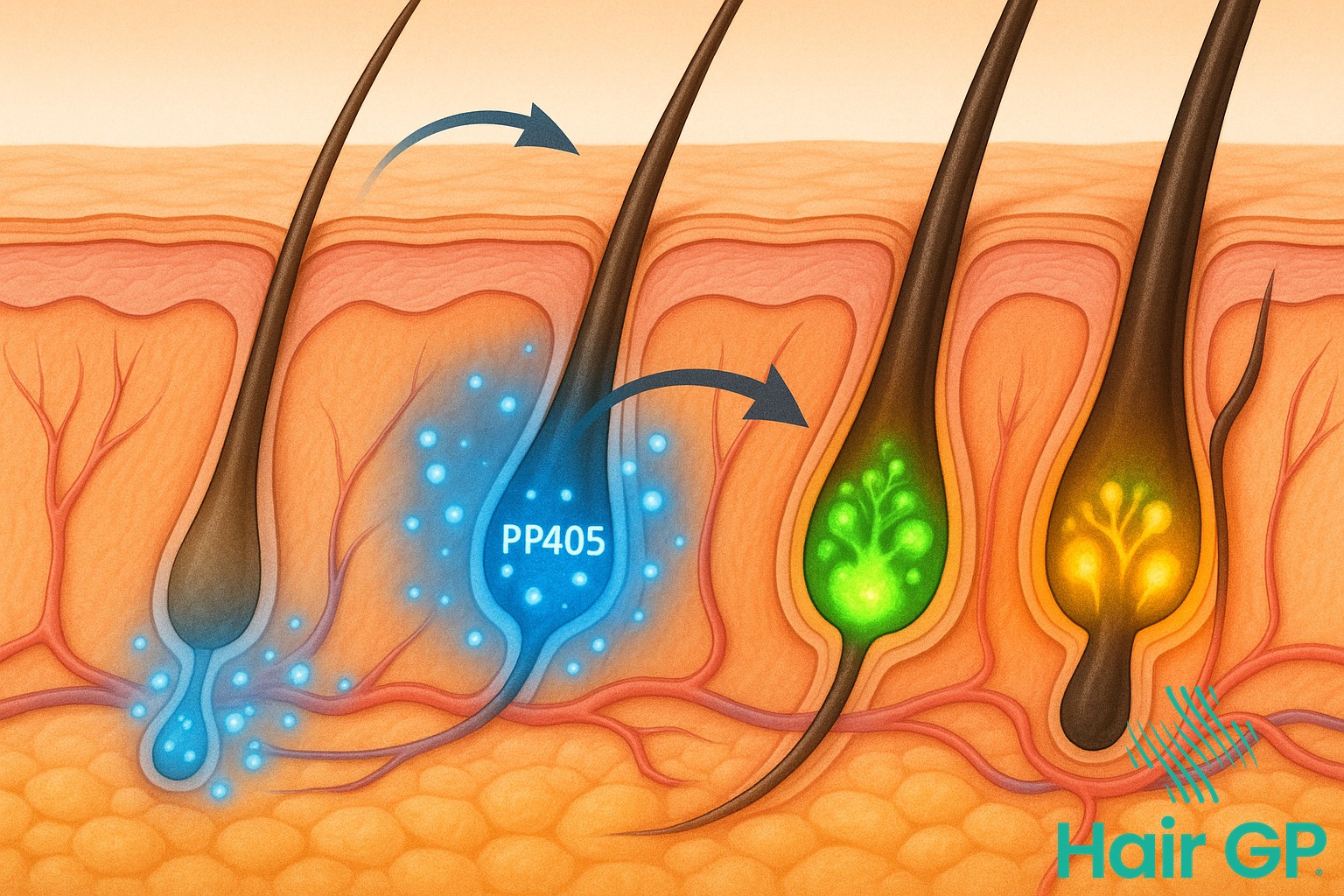
Hair follicles contain specialised stem cells that remain dormant during periods of hair loss. Research has shown that these cells retain their regenerative potential even in balding areas [1]. PP405 specifically targets these dormant stem cells, triggering a cascade of molecular signals that restore normal growth patterns.
The activation process begins when PP405 interacts with specific receptor proteins on follicle stem cells. This interaction stimulates the production of growth factors essential for hair regeneration. Unlike conventional treatments that merely support existing follicles, PP405 fundamentally reactivates the scalp’s natural regenerative mechanisms, offering unprecedented potential for complete hair restoration.
The Hair Growth Cycle and PP405
Human hair growth follows a precise cycle consisting of anagen (growth), catagen (transition), and telogen (resting) phases. In pattern baldness, follicles become stuck in extended telogen phases, producing progressively thinner hairs before ceasing production entirely [2].
PP405 disrupts this destructive pattern by extending the anagen phase whilst simultaneously reducing telogen duration. This optimisation allows follicles to produce thicker, healthier hairs for longer periods. The treatment’s ability to synchronise follicle cycles across affected areas ensures uniform hair growth, avoiding the patchy results common with other therapies. Through this comprehensive approach to cycle regulation, PP405 transforms the fundamental science of hair restoration.
Clinical Trials and Results: What the Data Shows
The clinical development of PP405 represents a significant milestone in hair restoration research, with early human trials demonstrating promising results within an unprecedented timeframe. Initial testing has focused on establishing both safety and efficacy parameters, with particular attention to the rapid onset of action that distinguishes this treatment from existing therapies.
First Human Trials: Breakthrough Results
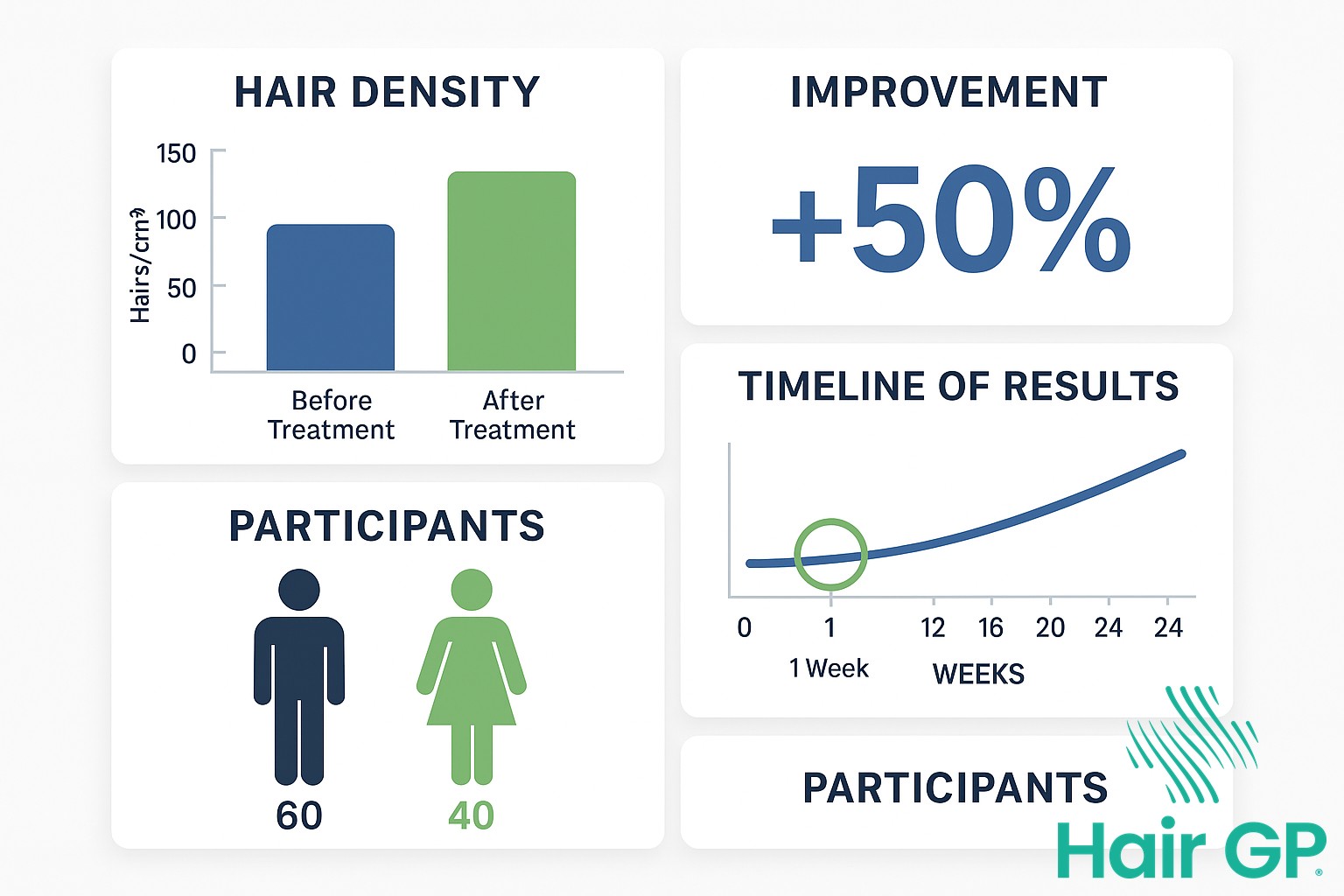
The first human trials of PP405 employed a rigorous double-blind, placebo-controlled design to evaluate treatment outcomes. Participant selection criteria included adults aged 18-65 with androgenetic alopecia, representing diverse ethnic backgrounds and hair loss patterns. The trial protocol involved daily topical application with assessments conducted at baseline and weekly intervals.
Results from the initial week produced promising results that exceeded researchers’ expectations. Participants demonstrated measurable improvements in follicle activation markers, with imaging studies revealing early signs of stem cell mobilisation. These findings were deemed statistically significant when compared to placebo controls, suggesting genuine biological activity rather than chance variation.
Future Clinical Studies and Timeline
Building on these encouraging results, Phase II clinical trials are currently in planning stages. These expanded studies will involve larger participant cohorts and extended treatment durations to establish optimal dosing regimens and long-term safety profiles. The regulatory pathway includes comprehensive safety evaluations and efficacy benchmarking against current standard treatments.
Market timeline projections suggest potential availability within 3-5 years, pending successful completion of regulatory requirements. This accelerated development schedule reflects both the urgent medical need and the promising early-stage data supporting PP405’s therapeutic potential.
PP405 vs. Current Hair Loss Treatments
When evaluating PP405 against established hair loss treatment options, several key differences emerge. Traditional hair transplants offer permanent results but involve surgical procedures and recovery time. Platelet rich plasma therapy utilises the body’s own healing factors, though multiple sessions are typically required. Whilst medications like finasteride and minoxidil have proven effective for many, they often necessitate indefinite use to maintain results. PP405’s protein-based approach presents a novel alternative that stimulates natural regeneration without surgery or continuous medication. Each treatment serves different patient needs, with PP405 potentially bridging gaps between non-invasive therapies and more permanent solutions, offering unique advantages in the evolving landscape of hair restoration.
Conclusion
PP405 represents a transformative breakthrough that could redefine the future of hair restoration. Unlike existing treatments that merely slow hair loss, this innovative molecule’s ability to activate dormant follicle stem cells offers genuine promise for those seeking a lasting solution. The remarkable results from initial human trials, showing significant improvement within just one week, provide tangible hope to millions worldwide who struggle with hair loss and its psychological impact.
Whilst we must temper expectations—PP405 is not yet a guaranteed cure—the science behind this treatment fundamentally changes our understanding of baldness treatment. Its molecular approach addresses the root cause rather than symptoms, potentially offering more permanent results than current options. The journey from laboratory to pharmacy shelf requires patience, with further trials and regulatory approvals ahead.
Nevertheless, the promise of PP405 cannot be overstated. For the first time in decades, we’re witnessing a treatment that could genuinely restore hair growth rather than simply preventing further loss. As research continues and trials expand, this breakthrough offers hope that effective hair restoration may soon become reality for countless individuals. The future of treating hair loss has never looked more promising.
Frequently Asked Questions
Based on current clinical trial progress, PP405 is expected to undergo further trials over the next 2-3 years. If these trials continue to show positive results, Pelage Pharmaceuticals estimates market availability could occur within 3-5 years, pending regulatory approval.
While initial trials have shown promising results, PP405 is primarily designed to activate dormant follicle stem cells. It may be most effective for pattern baldness and thinning hair where follicles are still present but inactive. Further studies will determine its effectiveness for various hair loss conditions.
Early clinical trials have not reported significant adverse effects, but comprehensive safety data is still being collected. As with any new treatment, full safety profiles will be established through extensive clinical testing before public availability.
While pricing has not been officially announced, PP405 is expected to be more affordable than surgical hair transplants since it’s a pharmaceutical treatment rather than a surgical procedure. Exact costs will depend on treatment duration and dosing requirements.
References
- Wang L, Siegenthaler JA, Dowell RD, Yi R. Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science. 2016;351(6273):613-617. PMID: 26912704
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502(7472):513-518. PMID: 24097351